-
Новости
- ИССЛЕДОВАТЬ
-
Страницы
-
Группы
-
Мероприятия
-
Reels
-
Статьи пользователей
-
Offers
-
Jobs
-
Форумы
-
Кинозал
Plasmid DNA Manufacturing Market Size, Growth, Trends, Forecast (2024-2032)
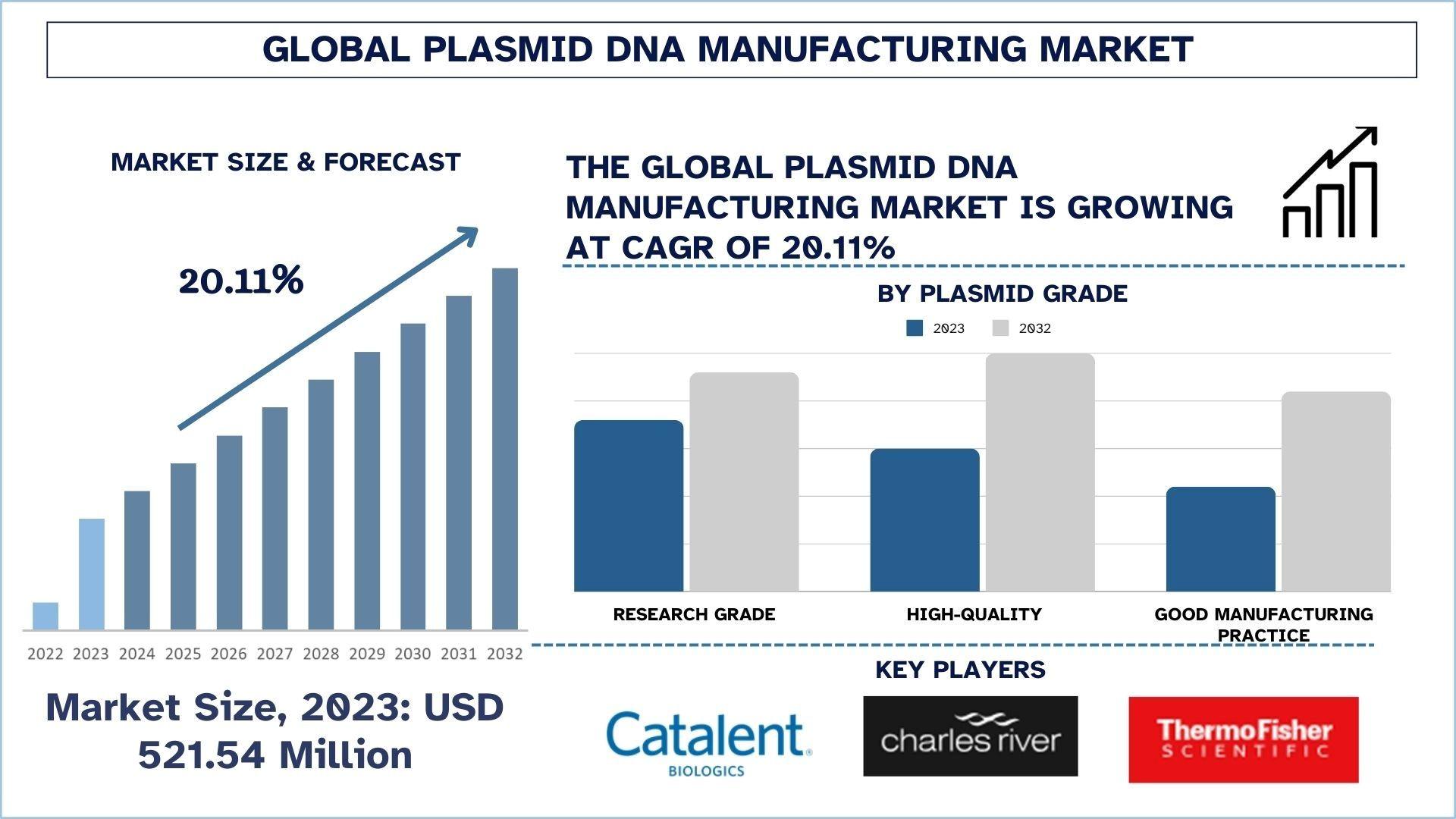
According to the UnivDatos, the rise in prevalence of cancer, and infectious diseases, increasing demand for gene therapies, expansion of combination therapy, and increasing focus towards research and development drive the Plasmid DNA manufacturing market. As per their “Plasmid DNA Manufacturing Market” report, the global market was valued at USD 521.54 million in 2023, growing at a CAGR of about 20.11% during the forecast period from 2024 - 2032 to reach USD million by 2032.
Increasing Collaboration Activities are driving high demand in North America
North American plasmid DNA manufacturing is one of the leading markets in the world. This growth is a result of the rising need for gene therapies, genetic vaccines, and better and superior medical treatments, especially for diseases that are becoming more and more prevalent throughout the globe. With important investments in biotechnology and a qualified population with adequate access to healthcare services, North America is showing tremendous growth. Furthermore, impressive partnerships between market stakeholders and universities are helping to drive innovation further into plasmid-based treatments and vaccines.
For instance, in May 2024, Siren Biotechnology, a United States-based company, entered a strategic partnership with Catalent Inc., the leader in enabling the development and supply of better treatments for patients worldwide, to support the development and manufacturing of Siren Biotechnology’s AAV immuno-gene therapies.
Growing Plasmid DNA Manufacturing Facilities in the United States
The United States is one of the largest markets for plasmid DNA in the world. Some of the factors contributing to the expansion of the market include the growing number of cancer and genetic disorder cases which require better treatment methods and more manufacturing facilities. The US has a wide array of biopharmaceutical firms and CDMOs that provide GMP services, which improves the country’s competitiveness within the industry. Furthermore, any favorable governmental policies that seek to promote gene therapies also fuel the uptake of plasmid DNA products. This growth also improves patient care through the availability of new therapeutic approaches while placing companies based in the United States at the cutting edge of biopharmaceutical development worldwide.
For instance, in July 2022, Thermo Fisher Scientific Inc., the world leader in serving science, opened a new cGMP plasmid DNA manufacturing facility in Carlsbad, California, enabling it to meet rapidly growing demand for plasmid DNA-based therapies and vital mRNA-based vaccines.
Growing Biopharmaceutical Industry is driving high demand in Canada
Plasmid DNA manufacturing in Canada is still a developing industry and is growing massively. This development is caused by the increase in healthcare expenditure and the development of the pharmaceutical market based on new therapeutic methods. Canada has a well-developed research infrastructure consisting of academic and knowledge-based organizations working with biotechnology companies to develop R&D projects with relevance to therapeutic applications of plasmids. As Canada increases its capacity to develop plasmid DNA for therapeutics, the country further strengthens its standing as a biopharmaceutical player in the global market. It can provide better health for the people by developing new therapies for the consistent genetic diseases that are found widespread in the Canadian people, as well as, encouraging foreign investments to the local biotechnology-based companies.
Access sample report (including graphs, charts, and figures) - https://univdatos.com/reports/plasmid-dna-manufacturing-market?popup=report-enquiry
For instance, in June 2024, Entos Pharmaceuticals, a clinical-stage biotechnology company developing genetic medicines with its proprietary Fusogenix PLV nucleic acid delivery platform, and its partner, Aegis Life, Inc., announced that Entos has received approval from Health Canada to initiate a phase 1/2 clinical trial evaluating Covigenix VAX-002, an investigational COVID-19 booster vaccine, which is a plasmid DNA vaccine.
Conclusion
The market in North America has great growth potential because the region has a high prevalence and incidence of cancer. The availability of the advanced biotechnology industry, favorable regulatory structures, growing research, and development, along with increased collaboration activities influence the market’s growth. North America's commitment to bringing innovation in this market further escalates North America’s position as the leading region for the plasmid DNA manufacturing market.
Contact Us:
Email - contact@univdatos.com
Website - www.univdatos.com
- Plasmid_DNA_Manufacturing_Market
- Plasmid_DNA_Manufacturing_Market_Size
- Plasmid_DNA_Manufacturing_Market_Growth
- Plasmid_DNA_Manufacturing_Market_Trends
- Plasmid_DNA_Manufacturing_Market_Forecast
- Plasmid_DNA_Manufacturing_Market_Share
- Plasmid_DNA_Manufacturing_Market_Reports
- Plasmid_DNA_Manufacturing_Market_Segment
- AI
- Vitamins
- Health
- Admin/office jobs
- News
- Art
- Causes
- Crafts
- Dance
- Drinks
- Film
- Fitness
- Food
- Игры
- Gardening
- Health
- Главная
- Literature
- Music
- Networking
- Другое
- Party
- Religion
- Shopping
- Sports
- Theater
- Wellness


