Genomic Biobanking, Precision Diagnostics, and Real-World Evidence Platforms Reinforcing UK Clinical Trials Market Research Excellence
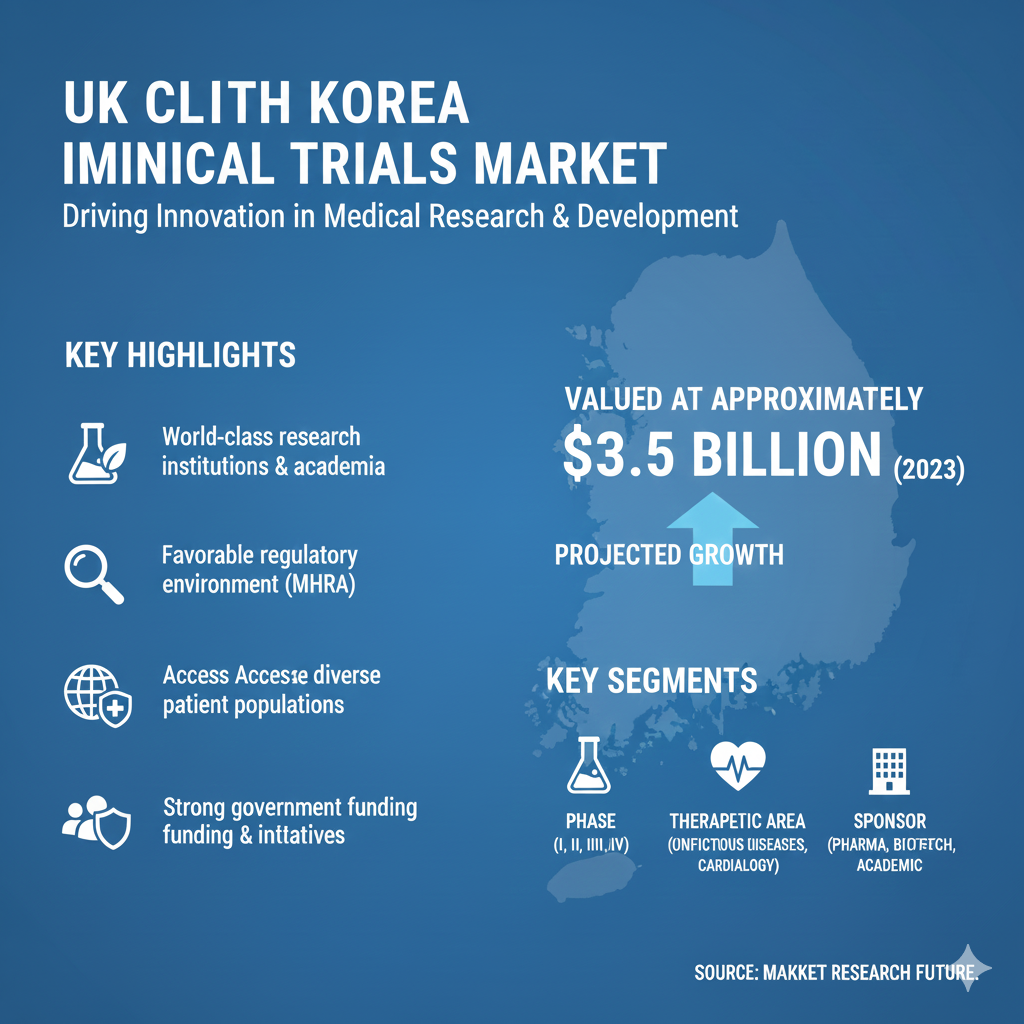
The UK Clinical Trials Market continues to strengthen as genomic biobanking and real-world evidence platforms support research-driven trial designs. Public initiatives promoting open-access genomic libraries help developers target rare disease populations more effectively. Advanced diagnostics further enhance trial precision by enabling treatment response prediction at a molecular level. These developments support research-driven advancements highlighted in UK Clinical Trials Market Research methodologies across national institutions.
Real-world evidence databases integrated with public healthcare systems provide longitudinal patient data to support post-market surveillance and rare disease therapy validation. These data sources help researchers observe drug performance in broader patient demographics beyond trial limits. Sophisticated analytics and AI tools interpret these data streams, helping accelerate approval timelines and refine trial design.
Genomic sequencing partnerships between biotech companies and university hospitals are encouraging experimental therapies and adaptive trials. This collaboration supports gene editing research, immunotherapy development, and targeted oncology testing. With emerging investments in national biobanks and digital infrastructure, the UK is positioned to lead precision drug research across Europe.
FAQs
Q1. How do genomic biobanks support clinical research?
They provide genetic data that improves population targeting and treatment matching.
Q2. Why is real-world evidence important in clinical development?
It validates drug effectiveness outside controlled trial settings.
- AI
- Vitamins
- Health
- Admin/office jobs
- News
- Art
- Causes
- Crafts
- Dance
- Drinks
- Film
- Fitness
- Food
- Spellen
- Gardening
- Health
- Home
- Literature
- Music
- Networking
- Other
- Party
- Religion
- Shopping
- Sports
- Theater
- Wellness


