EGFR-NSCLC Market Trends & Future Outlook, 2030 | UnivDatos
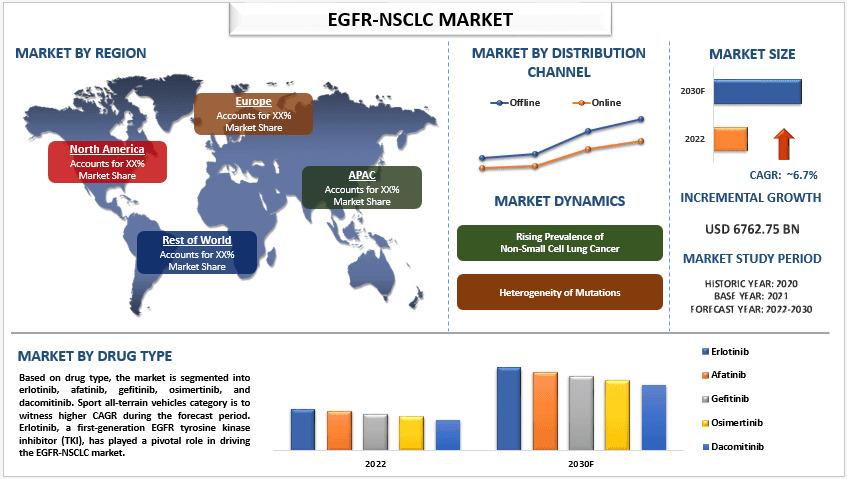
According to the UnivDatos, the development of advanced technologies would massively transform the global scenario of EGFR-NSCLC and as per their “EGFR-NSCLC Market” report, the global market was valued at USD 6762.75 million in 2021, growing at a CAGR of 6.7% during the forecast period from 2023 - 2030 to reach USD 11471.29 million by 2030.
The growth trajectory of the EGFR-NSCLC market is further propelled by escalating healthcare investments dedicated to oncology research and drug development. The oncology sector, including EGFR-NSCLC treatment, has consistently received substantial funding from both private investors and government agencies. In 2020 alone, global investments in oncology reached approximately USD18 billion, according to industry data. These investments empower pharmaceutical companies to accelerate drug discovery, conduct extensive clinical trials, and develop novel therapies targeting EGFR mutations in NSCLC. Additionally, healthcare systems are increasingly allocating resources to support the adoption of innovative targeted therapies, allowing more patients to access these advanced treatments. The confluence of rising healthcare investments and a strong clinical pipeline not only fosters the development of groundbreaking therapies but also fosters a comprehensive approach to improving patient outcomes in the EGFR-NSCLC market.
Access sample report (including graphs, charts, and figures): https://univdatos.com/reports/egfr-nsclc-market?popup=report-enquiry
Recent Developments/Awareness Programs:- Several key players and governments are rapidly adopting strategic alliances, such as partnerships, or awareness programs for the treatment:-
· on April 4, 2022, Janssen Pharmaceutical announced that Health Canada has issued a Notice of Compliance with Conditions (NOC/c) approving RYBREVANT (amivantamab), a fully human, bispecific antibody, for the treatment of adult patients with locally advanced or metastatic non-small cell lung cancer (NSCLC) with activating EGFR Exon 20 insertion mutations whose disease has progressed on or after platinum-based chemotherapy.
· In January 2023, announced that EXKIVITY (mobocertinib) has been approved by the National Medical Products Administration (NMPA) of China for the treatment of adult patients with locally advanced or metastatic non-small cell lung cancer (NSCLC) with epidermal growth factor receptor (EGFR) Exon20 insertion mutations, whose disease has progressed on or after platinum-based chemotherapy.
Click here to view the Report Description & TOC: https://univdatos.com/reports/egfr-nsclc-market
The demand for Tagrisso has been boosted by several factors, including:
• The approval of Tagrisso by regulatory agencies, such as the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA), for the treatment of EGFRm NSCLC.
• The success of clinical trials that have shown the efficacy and safety of Tagrisso in treating EGFRm NSCLC.
• The availability of reimbursement and access programs have made Tagrisso more accessible to patients.
• The emergence of other targeted therapies for NSCLC, has increased the overall demand for EGFR-targeted therapies in this market.
Conclusion:
In conclusion, the EGFR-NSCLC market stands at the forefront of precision medicine, bringing hope to patients and healthcare professionals alike. As targeted therapies continue to evolve and new advancements are made in the understanding of EGFR mutations, the landscape of treatment options is expanding rapidly. With the promise of improved efficacy, reduced side effects, and personalized care, the future of EGFR-NSCLC treatment holds immense potential. However, challenges such as drug resistance and accessibility need to be addressed to ensure that these innovations reach a wider patient population. By fostering collaboration between researchers, pharmaceutical companies, and healthcare providers, we can collectively propel the EGFR-NSCLC market toward greater success and better outcomes for those battling this complex disease.
Contact Us:
UnivDatos
Contact Number - +1 978 733 0253
Email - contact@univdatos.com
Website - www.univdatos.com
Linkedin- https://www.linkedin.com/company/univ-datos-market-insight/mycompany/
- AI
- Vitamins
- Health
- Admin/office jobs
- News
- Art
- Causes
- Crafts
- Dance
- Drinks
- Film
- Fitness
- Food
- Giochi
- Gardening
- Health
- Home
- Literature
- Music
- Networking
- Altre informazioni
- Party
- Religion
- Shopping
- Sports
- Theater
- Wellness


