-
Новости
- ИССЛЕДОВАТЬ
-
Страницы
-
Группы
-
Мероприятия
-
Reels
-
Статьи пользователей
-
Offers
-
Jobs
-
Форумы
-
Кинозал
Advanced Bacterial Reverse Mutation Test (Ames) Service to Accelerate Medical Device Safety Assessment
STEMart, a US-based provider of comprehensive services for all phases of medical device development, announced the expansion of its service offerings to include the Bacterial Reverse Mutation Test (Ames) service. This service provides manufacturers and researchers a rapid, cost-effective, and robust method for assessing the mutagenic potential of new chemicals and leachable substances from medical devices, in compliance with the guideline of OECD 471.
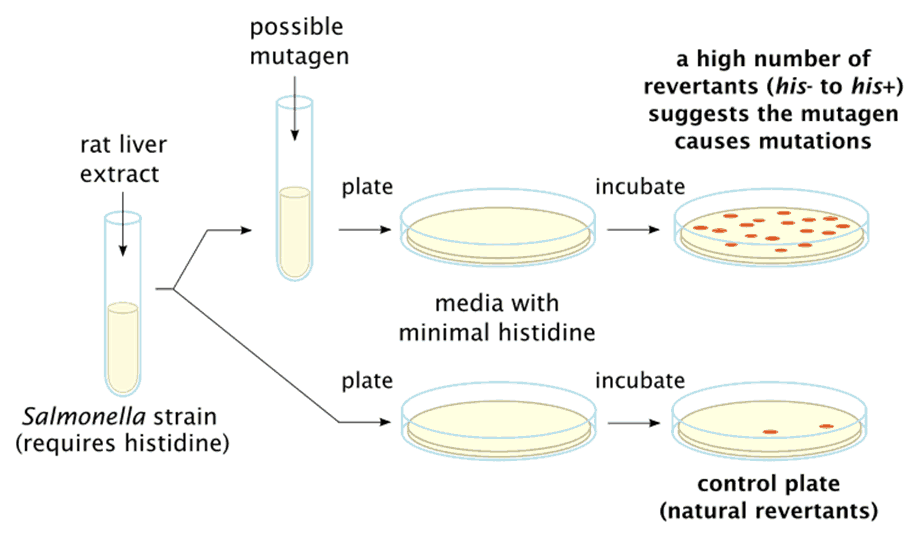
The bacterial reverse mutation test, commonly known as the Ames test, is a widely used method for detecting bacterial gene mutations. It is favoured for its simplicity, accuracy and low cost. The assay measures the number of colonies formed after exposure to the test chemical. If the bacteria have undergone mutations, the number of colonies formed will be significantly higher than in the negative control cultures. This method is highly sensitive to most genotoxic carcinogens.
As a primary screening tool, the Ames test can assess the mutagenic potential of novel chemicals and pharmaceuticals. It is also a mandatory test when submitting registration or approval data to regulatory authorities for diverse chemicals, including pharmaceuticals and biocides. International organizations have established standardized guidelines to ensure that companies and testing laboratories adhere to proper operational procedures.
Conducted in accordance with OECD Guidelines for Testing of Chemicals, Test No. 471, the bacterial reverse mutation test (Ames) uses histidine-dependent strains of Salmonella typhimurium or Escherichia coli to detect permanent gene mutations (e.g., single or multiple base pair substitutions, insertions or deletions) caused by new chemicals and candidate drugs. To support the medical device industry, STEMart now provides bacterial reverse mutation test services to evaluate the mutagenic potential of substances leaching from medical devices. This testing can be carried out under GLP (Good Laboratory Practice) or non-GLP conditions.
STEMart's Ames test evaluates a substance's mutagenic potential using at least five bacterial strains, including Salmonella typhimurium (e.g., TA97, TA98, TA100, TA102, TA1535 and TA1537) with mutations in the histidine operon and E. coli (e.g., E. coli uvrA and E. coli pKM101) with mutations in the tryptophan operon. The number and set of strains used can be determined based on regulatory requirements.
Health regulatory authorities consider this new bacterial reverse mutation test to be the gold standard for predicting a substance's potential to cause cancer in humans. It is highly sensitive and can detect suitable mutants in a wide range of bacteria. A key advantage of the Ames test is its ability to distinguish between direct-acting mutagens and pro-mutagens (substances that become mutagenic after being metabolized). Furthermore, the specificity of the test strains can provide valuable insights into the types of mutations induced by genotoxic agents.
STEMart's final reports include a detailed methodology, raw data, analysis and clear results interpretation, ensuring full transparency for clients. To find out more about other medical device testing solutions, or to consult with the experts at STEMart, please visit https://www.ste-mart.com/bacterial-reverse-mutation-test-ames.htm.
About STEMart
STEMart is an industry-leading eCommerce platform incorporated with an extensive global footprint and a broad portfolio of more than 10,000 products. STEMart aims to provide better lab materials, medical instruments and consumables, excellent technologies, and high-quality services to global customers in the fields of science, technology, and engineering, from the discovery stage to manufacturing. STEMart is dedicated to enhancing research and biotech production by providing simpler and safer protocols for better health worldwide.
- AI
- Vitamins
- Health
- Admin/office jobs
- News
- Art
- Causes
- Crafts
- Dance
- Drinks
- Film
- Fitness
- Food
- Игры
- Gardening
- Health
- Главная
- Literature
- Music
- Networking
- Другое
- Party
- Religion
- Shopping
- Sports
- Theater
- Wellness


