Virtual Clinical Trials Market Size, Share And Growth Report, 2033
The global virtual clinical trials market is projected to grow at a CAGR of 4.67% during the forecast period 2025-2033. The market size was valued at USD 3899 billion in 2024. Key growth drivers include patient-centric approaches, technological advancements such as telemedicine and wearables, the accelerated adoption due to the COVID-19 pandemic, and increasing regulatory acceptance.
The Virtual Clinical Trials Market Trends highlight a growing shift toward decentralized and digitally enabled research models in the healthcare industry. Pharmaceutical and biotechnology companies are increasingly using virtual clinical trials to reduce costs, speed up patient recruitment, and improve participant convenience. By leveraging technologies such as wearable devices, mobile apps, telemedicine, and remote data monitoring, trials can be conducted with fewer site visits while maintaining data accuracy and compliance. These approaches also help reach more diverse patient populations and improve retention rates. As digital health tools continue to advance and regulatory support grows, virtual clinical trials are becoming a practical and efficient alternative to traditional trial methods, shaping the future of clinical research.
Study Assumption Years
- Base Year: 2024
- Historical Year/Period: 2019-2024
- Forecast Year/Period: 2025-2033
Virtual Clinical Trials Market Key Takeaways
- Current Market Size: USD 3899 billion (2024)
- CAGR: 4.67%
- Forecast Period: 2025-2033
- The virtual clinical trials market is driven by the increasing emphasis on patient-centric trials and the need for reduced drug development cycles.
- Technological improvements in telemedicine, wearables, and mobile health apps facilitate remote patient monitoring, decreasing the necessity for physical site visits.
- The COVID-19 pandemic demonstrated virtual trials' capability to maintain study performance amidst restrictions, accelerating market growth.
- Market trends highlight integrated platforms offering complete virtual trial solutions from patient recruitment to data analytics, with rising use of AI and ML for efficiency.
- North America is the dominant regional market, benefiting from advanced healthcare IT infrastructure, regulatory support, and a broad patient pool.
Download a sample PDF of this report: https://www.imarcgroup.com/virtual-clinical-trials-market/requestsample
Market Growth Factors
The virtual clinical trials market is primarily driven by the growing emphasis on patient-centric clinical trials. Traditional trials often face challenges with patient recruitment, engagement, and retention due in part to the inconvenience of site visits. Virtual trials focus on patient comfort, convenience, and accessibility, enabling patients to participate from their homes, thus overcoming transportation and scheduling challenges. This patient-focused approach enhances inclusivity and engagement, driving market growth.
Technological advancements play a critical role in supporting the virtual clinical trials market. Specifically, telemedicine facilitates remote consultations between patients and physicians, ensuring constant communication and support throughout the trial duration. Wearable devices enable 24/7 monitoring of vital signs and other health parameters, providing researchers with real-time data. Additionally, remote monitoring systems promote data accuracy by reducing human error in manual documentation. These technologies collectively improve trial efficiency and patient data quality, further propelling market adoption.
The COVID-19 pandemic significantly accelerated the adoption of virtual clinical trials. With traditional trials disrupted by shutdowns and social distancing measures, the pandemic underscored the vulnerability of centralized trial models. Virtual trials allowed research continuity by enabling remote patient participation and data collection, maintaining study integrity despite restrictions. This pivot to virtual approaches during the pandemic stimulated innovation and wider acceptance of virtual trials in the industry, which continues to drive market expansion.
Market Segmentation
Breakup by Study Design:
- Interventional: Represents the largest segment driven by the demand for innovative therapies, enabling streamlined participant recruitment, accelerated drug evaluation, broader geographical reach, and tailored patient interventions.
- Observational: Not separately described in detail beyond segment name.
- Expanded Access: Not separately described in detail beyond segment name.
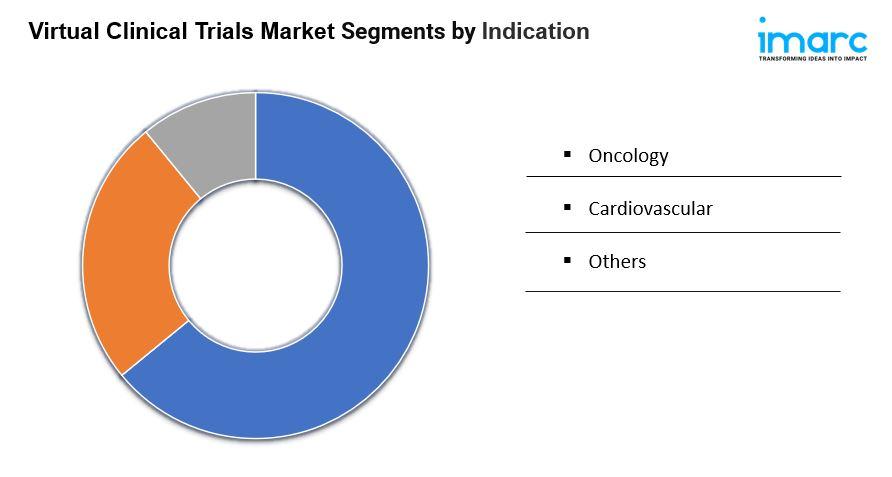
Breakup by Indication:
- Oncology: Largest segment due to the increasing global cancer prevalence, advances in targeted therapies and precision medicine, and demand for specialized oncology trials addressing rare and genetically-defined cancers.
- Cardiovascular: Not separately described in detail beyond segment name.
- Others: Not separately described in detail beyond segment name.
Breakup by Region:
- North America: Largest market share attributed to advanced healthcare IT infrastructure, telemedicine adoption, favorable regulations, strong clinical R&D focus, and a broad patient population.
- United States
- Canada
- Asia Pacific: China, Japan, India, South Korea, Australia, Indonesia
- Europe: Germany, France, United Kingdom, Italy, Spain, Russia
- Latin America: Brazil, Mexico
- Middle East and Africa
Regional Insights
North America is the dominant regional market for virtual clinical trials, driven by a strong healthcare IT infrastructure, high adoption of digital health technologies like telemedicine and wearables, and favorable regulatory guidelines. The region benefits from a broad patient pool and significant clinical research activity, particularly in the United States, which accelerates the deployment of virtual trial platforms. These factors collectively support the largest market share and strong forecast growth in North America.
Recent Developments & News
In June 2022, Medidata announced the addition of ten innovative organizations to its Sensor Cloud Network, focusing on solving challenges related to sensor integration, data standardization, and digital biomarker development. Participating organizations include AliveCor, Aural Analytics, Biobeat, and several universities.
In June 2021, Veradigm, a unit of Allscripts Healthcare Solutions, and PRA Health Sciences announced the creation of the leading electronic healthcare records-based clinical research network, reaching over 25,000 physicians and 40 million US patients, enhancing clinical research capabilities.
Key Players
- Clinical Ink Inc.
- Covance Inc.
- ICON Plc
- IQVIA Inc.
- LEO Innovation Lab
- Medable Inc.
- Medidata Solutions Inc. (Dassault Systèmes SE)
- Medpace Holdings Inc.
- Oracle Corporation
- Parexel International Corporation (Pamplona Capital Management)
- PRA Health Sciences
- Signant Health (Genstar Capital)
Customization Note:
If you require any specific information that is not covered currently within the scope of the report, we will provide the same as a part of the customization.
Ask An Analyst: https://www.imarcgroup.com/request?type=report&id=3221&flag=C
About Us
IMARC Group is a global management consulting firm that helps the world’s most ambitious changemakers to create a lasting impact. The company provide a comprehensive suite of market entry and expansion services. IMARC offerings include thorough market assessment, feasibility studies, company incorporation assistance, factory setup support, regulatory approvals and licensing navigation, branding, marketing and sales strategies, competitive landscape and benchmarking analyses, pricing and cost research, and procurement research.
Contact Us
IMARC Group,
134 N 4th St. Brooklyn, NY 11249, USA,
Email: sales@imarcgroup.com,
Tel No: (D) +91 120 433 0800,
United States: +1-201971-6302
- AI
- Vitamins
- Health
- Admin/office jobs
- News
- Art
- Causes
- Crafts
- Dance
- Drinks
- Film
- Fitness
- Food
- Jogos
- Gardening
- Health
- Início
- Literature
- Music
- Networking
- Outro
- Party
- Religion
- Shopping
- Sports
- Theater
- Wellness



